News
FAQ: What You Need To Know About Pfizer’s COVID Vaccine And Adolescents
By: Pien Huang | NPR
Posted on:
WASHINGTON, D.C. (NPR) — Teens and preteens in the U.S. have spent much of the past year distance learning. Many have missed out on birthday parties, book clubs, team sports and hanging out with groups of friends.
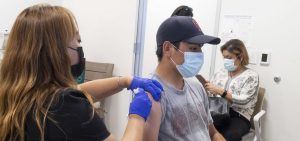
The authorization expands the pool of eligible vaccine recipients to about 87% of the total U.S. population, covering an additional 17 million children, and comes at a time when people under age 18 account for one 1 of every 5 newly reported coronavirus infections.
The ability to get vaccinated is crucial for this group, Dr. Nia Heard-Garris, a pediatrician and researcher at Northwestern University’s School of Medicine, tells NPR. “It promotes the potential to gather and socialize and continue on — which is just as important for children as for adults.”
Kids and their parents have lots of questions. Here’s what we’ve learned so far:
When can this group start getting their shots?
A vaccine advisory committee to the Centers for Disease Control and Prevention is scheduled to meet Wednesday to make recommendations about how the vaccine should be used. It’s expected that the shots will be widely available to 12- to 15-year-olds as early as this week.
Is the number of doses or timing of this vaccine different for this age group than for adults?
No, everything about the dosing is the same: two shots of the Pfizer vaccine for full vaccination — each scheduled about 21 days apart. Previous studies of other vaccines, as well the clinical research on Pfizer’s COVID-19 vaccine, confirm the dosing regimen should work well in this age group, providing robust immunity with few side effects.
How do we know it works in kids?
In the vaccine clinical trial, there were no cases of COVID-19 in the 1,100 children who received the Pfizer vaccine and 16 cases in the 1,100 children in the placebo group, according to the FDA. The trial also found that vaccinated adolescents had high levels of antibodies in their blood — a signal they had developed strong protective immunity.
“The vaccine was 100% effective in preventing COVID-19,” the FDA announced Monday. “At this time, data are not available to determine how long the vaccine will provide protection.”
What about side effects in this group?
“A lot of [the people in the 12-15 age group] did have similar side effects to the young adults, with the second dose in particular,” says Dr. Megan Freeman, a researcher in infectious disease at UPMC Children’s Hospital of Pittsburgh.
Specifically, the clinical research found that many in this age group who received the vaccine reported temporary pain at the injection site (91%), and in the next day or so were tired (78%) or had a headache (76%). It was also common to experience chills (49%) or muscle pain (42%). Other, less frequent side effects reported included fever, joint pain and nausea.
“These vaccines are eliciting an immune response that can cause some local reactions, [including] low-grade fevers and flu-like symptoms,” says Dr. Yvonne Maldonado, chair of the Committee on Infectious Diseases for the American Academy of Pediatrics and a professor of pediatric infectious diseases at Stanford. “But those are short-lived and in the end, they provide an immune response for protection against disease.”
Why should kids this age get vaccinated?
Children and adolescents can get sick from infection with the coronavirus and they can infect others. And while, in general, their cases tend to be less severe, some children have developed serious complications.
“We know that kids are less likely to die from COVID than, say, their 80-year-old grandparents,” says Freeman. “But that doesn’t mean that there’s zero risk.”
In the United States alone, tens of thousands of kids have been hospitalized with COVID-19 — including more than 3,000 who have developed a rare but dangerous inflammatory syndrome nicknamed MIS-C. During the pandemic, COVID-19 has been one of the leading causes of death among children, Dr. Sean O’Leary of the American Academy of Pediatrics tells NPR — some 300 to 600 children have died.
There are also increasing concerns about persistent, long-term effects of the viral infection — such as fatigue, respiratory issues and stomach problems — for some children who get COVID-19. “We know that teenagers can get things like long COVID, and that’s something that you would want to avoid,” Freeman says. “Student athletes can have long-lasting effects on their heart and have to have monitoring by a cardiologist. So that would be something that we would want to avoid.”
And while most children who catch the coronavirus develop few or no symptoms, they can still, inadvertently, transmit the virus to others. “Because they are more likely to be asymptomatic, that transmission can be silent,” Maldonado says, so vaccinating kids “just makes it so much easier to assure that children are not being infected.”
In particular, vaccinating young teens “could be a big game changer,” O’Leary notes, “because we’ve known all along that adolescents tend to be both more likely to get infected and to spread the infection, relative to the younger kids. So getting that population vaccinated is also going to make a difference in these dynamics.”
Where will kids get their shots?
The Pfizer vaccine will be available to 12-year-olds and teens at most of the same places adults have been getting COVID-19 shots — plus at more drugstores and, in some cases, at their family doctor or pediatrician’s office.
On May 4, President Biden announced his administration was making special efforts to prepare retail pharmacies and pediatricians to give the vaccine to young people. “We are ready to move immediately … to make about 20,000 pharmacy sites across the country ready to vaccinate those adolescents as soon as the FDA grants its OK,” Biden said last week. “We’re also going to [ship] vaccines directly to pediatricians … so parents and their children can talk to their family doctor about it and get the shot from a provider they trust the most.”
Storage requirements and minimum dose requirements may make it harder for some small pediatric practices to give out the vaccine directly, notes Claire Hannan, head of the Association of Immunization Managers. “The vaccine requires a pharmacy-grade freezer, and many [doctors] have been hesitant to accept vials that contain [multiple] doses when they can’t guarantee they’re going to have that many people accept the vaccine once they open the vial.”
Some clinics may instead hold a big distribution day to get many kids vaccinated at once, Freeman says, while others may opt to refer young patients to a local pharmacy. The recently launched federal website Vaccines.gov is one place to check for locations near you.
The Biden administration is pushing states to encourage all teens to get their first Pfizer shots by July 4 — a timeline that would help make sure they’re fully vaccinated before school starts in the fall.
Will kids and teens have to prove their age or go with a parent to get the shot?
If the appointment is with a doctor’s office or a pharmacy the child has used before, they likely have an existing record of their age and insurance information, says Erin Fuse Brown, a health law professor at Georgia State University.
The question of proof of age becomes more pressing in community settings, such as drive-through or walk-up vaccination sites that don’t have a relationship with the minor, Fuse Brown says.
There’s recent precedent to draw from — teens 16 to 17 are currently eligible to get the Pfizer vaccine in all states, though most require that they get parental consent. And states have varying requirements over what counts as proof.
In New Hampshire, for example, according to the state’s COVID-19 hotline, a parent or guardian must accompany the minor getting the shot and bring official age documentation, such as a birth certificate or passport.
In California, some counties require minors to show up with a parent or legal guardian to get the vaccine, while others will accept a signed consent form.
In Maine, meanwhile, a parent vouching for their kid’s age through a written or verbal attestation will suffice. And the parent or guardian doesn’t have to be there for the shot — they can provide permission over the phone or sign a form beforehand.
Providers are checking for age and consent for good reason, says Fuse Brown: “They want to make sure they’re giving it to the population for whom it’s been authorized and in whom it’s been studied.”
Will there be settings where vaccination of this age group is required (for example, camps, sports or school)?
Maybe, though it’s very unlikely that the federal government will issue a national vaccination mandate, vaccine experts say.
Instead, whether kids must get vaccinated to participate in school and other communal activities will probably be decided on the state and local level.
“It’s all unknown at this point, but I can imagine that in some settings — for example, summer camps, perhaps, or even in some school districts or private schools — it may be likely that a vaccine will be required,” says Maldonado.
Already, some colleges are requiring COVID-19 vaccinations for students returning to campus this fall, and that could be a precedent for younger age groups, Freeman says. “Private institutions have caught on to this as a potential strategy for keeping their populations safe. It does get a bit trickier when it’s a public institution.”
A recent survey led by researchers at Northeastern University found that 58% of U.S. parents support vaccination requirements for returning to school, a sentiment that seems to be trending upward, the scientists say.
Still, state vaccination requirements often make room for exemptions based on medical, religious and sometimes philosophical reasons, Heard-Garris notes, “so I imagine there’s going to be an opportunity or a space for those families that don’t want their kids to have the vaccine or don’t feel comfortable or whatever to opt out.”
When will children younger than 12 be eligible for COVID-19 vaccination?
There’s been no official word yet, but preliminary clinical studies are already underway in younger children, and there are some signs that the vaccine might be available to them in the U.S. as early as this fall.
On a May 4 call with investors, Pfizer CEO Albert Bourla said his company plans to submit the applications for emergency use authorization in children ages 2-5 and 5-11 to the FDA in September.
Maldonado is running one of the several sites around the country enrolling children under 12 in research trials. “We will be looking very carefully at dosing and reactions to the vaccine and side effects in the very young children,” she says.
According to Bourla, Pfizer also plans to share the results of studies on the safety and efficacy of the vaccine in children ages 6 months to 2 years by the end of the year.
Kids and teens may also have a choice of COVID-19 vaccine in the near future. So far, the other two COVID-19 vaccines available in the U.S. — made by the drug companies Moderna and Johnson & Johnson — are restricted to adults ages 18 and up. But both companies are studying their vaccine in younger teens and children as well.
Having a COVID-19 vaccine available for adolescents is “really a remarkable achievement,” Maldonado says. “This will protect our children from the disease,” she says, “and if we want to get close to protection for the whole population, children are going to need to be a part of that.”
9(MDI4ODU1ODA1MDE0ODA3MTMyMDY2MTJiNQ000))

