News
A study indicates that an Alzheimer’s drug shows modest success slowing declines in memory, thinking
By: Jon Hamilton | NPR
Posted on:
WASHINGTON, D.C. (NPR) — An experimental drug that removes a substance called amyloid from the brain appears to slow down Alzheimer’s disease.
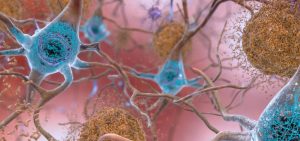
The study was published simultaneously in The New England Journal of Medicine.
People who got infusions of lecanemab scored about half a point better on a zero-to-18-point scale of mental functioning, a slight but statistically significant difference.
The results are “real and robust,” says Dr. Christopher van Dyck, who directs the Yale Alzheimer’s Disease Research Center and presented an overview of the study at the meeting.
But Dr. Madhav Thambisetty of the National Institute on Aging, who was not involved in the study, called the results “a very small effect.”
“It’s very unlikely that these differences are going to be noticeable by individual patients in their everyday lives,” Thambisetty says.
Thambisetty emphasized that his views are his own, and that he is not speaking for the NIA, which is part of the National Institutes of Health.
About one in five people who got lecanemab in the study experienced an adverse event, such as swelling or bleeding in the brain. People also reported symptoms including headaches, visual disturbances, and confusion.
The treatment has been linked to two deaths.
But most side effects are “mild to moderate,” says Dr. Marwan Sabbagh of the Barrow Neurological Institute, who gave a presentation on lecanemab’s safety. And the number of abnormalities detected on brain scans was “within expectations,” he says.
Even so, lecanemab is “not a benign drug,” Thambisetty says, adding that its risks may outweigh its benefits for some patients.
Lecanemab is being developed by the Japanese company Eisai along with the U.S. company Biogen.
The apparent success of lecanemab comes after many years of frustration and failure for companies developing drugs designed to clear amyloid from the brain.
So far, only one amyloid drug, Aduhelm, has received approval from the Food and Drug Administration.
That drug, also developed by Eisai and Biogen, was approved in 2021 despite conflicting evidence about whether it worked, and after an FDA advisory committee voted against approval.
Sales of Aduhelm have been slow, largely because Medicare will only cover the drug for patients participating in a clinical trial.
But Alzheimer’s patients and their families are already anticipating the arrival of lecanemab, despite its limitations.
“I’m a person living with a progressive and fatal disease,” says Michael Zuendel, 68, who has been taking Aduhelm since he was diagnosed with mild cognitive impairment, an early stage of Alzheimer’s. “I do not have time to wait for the perfect research study.”
“I’m extremely hopeful that the FDA will approve [lecanemab],” Zuendel says.
The Food and Drug Administration is expected to make a decision by January 6, 2023.
9(MDU1ODUxOTA3MDE2MDQwNjY2NjEyM2Q3ZA000))

