You are viewing the "Pfizer" Archives
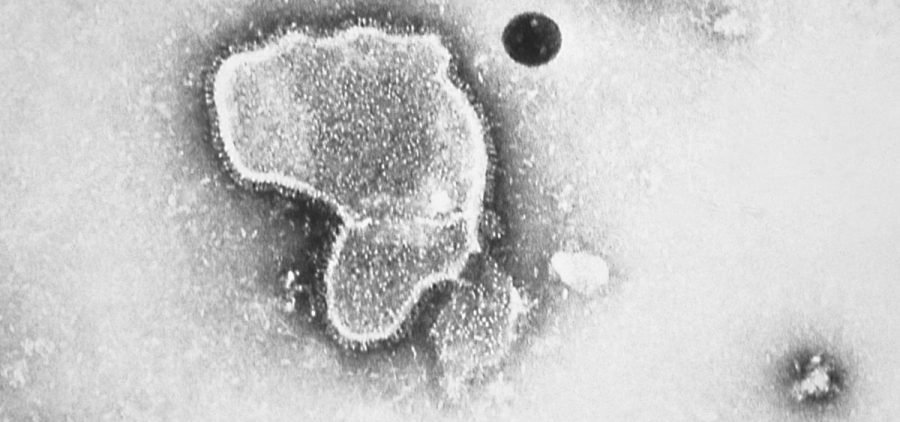
Pfizer’s RSV vaccine to protect babies gets greenlight from FDA
By: Sydney Lupkin | NPR
Posted on:
WASHINGTON (NPR) — The Food and Drug Administration has approved the first RSV vaccine for expectant mothers aimed at protecting their newborn babies. Given during the third trimester of pregnancy,… Read More
Pfizer asks FDA to greenlight new omicron booster shots, which could arrive this fall
By: Pien Huang | Will Stone | NPR
Posted on:
WASHINGTON, D.C. (NPR) — The U.S. is one step closer to having new COVID-19 booster shots available as soon as this fall. On Monday, the drugmakers Pfizer and BioNTech announced… Read More
Pfizer asks FDA to authorize booster shots for kids ages 5 through 11
By: Rob Stein | NPR
Posted on:
WASHINGTON, D.C. (NPR) — Children ages 5 through 11 who’ve received two shots of the Pfizer COVID-19 vaccine may soon be eligible for a booster. That’s if the Food and… Read More
The FDA OKs another Pfizer or Moderna COVID booster for people 50 and up
By: Associated Press
Posted on:
WASHINGTON, D.C. (AP) — U.S. regulators are allowing people 50 and older to get another booster dose of the Pfizer or Moderna COVID-19 vaccine. The Food and Drug Administration’s decision aims… Read More

The CDC now recommends Pfizer boosters after 5 months, down from 6
By: Becky Sullivan | NPR
Posted on:
WASHINGTON, D.C. (NPR) — People who were initially immunized with two shots of the Pfizer-BioNTech COVID-19 vaccine should receive a booster shot after five months, rather than six, according to… Read More
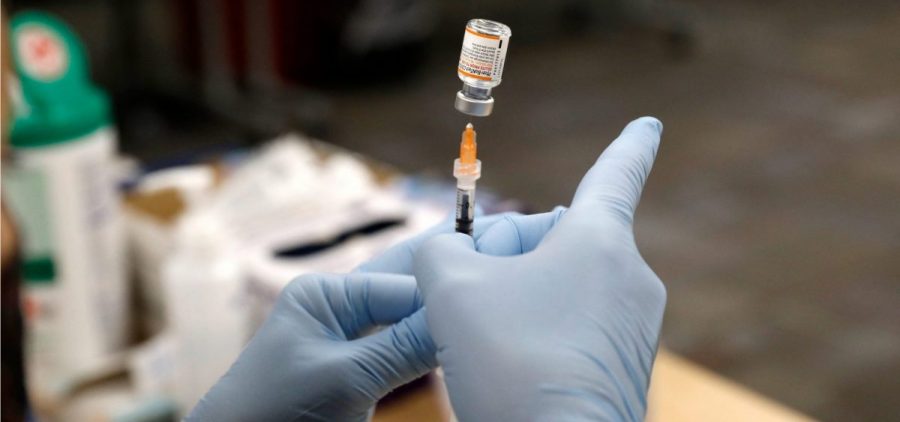
FDA authorizes a Pfizer booster shot for children ages 12 to 15
By: Scott Hensley | Joe Hernandez | NPR
Posted on:
Updated January 3, 2022 at 10:41 AM ET WASHINGTON, D.C. (NPR) — The Food and Drug Administration has authorized the use of a Pfizer-BioNTech booster in adolescents 12 to 15… Read More
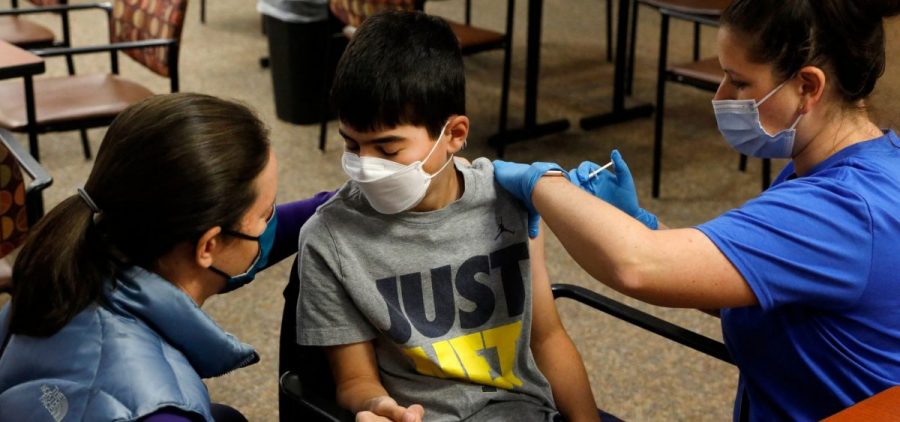
FDA authorizes use of Pfizer’s COVID vaccine for 5- to 11-year-olds
By: Joe Neel | NPR
Posted on:
WASHINGTON, D.C. (NPR) — The Food and Drug Administration has authorized a Pfizer-BioNTech COVID-19 vaccine for children ages 5 to 11. This lower-dose formulation of the companies’ adult vaccine was… Read More
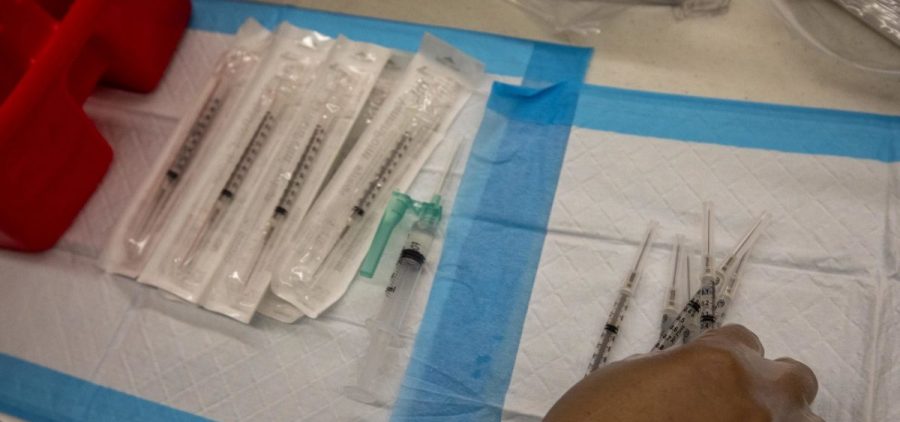
Pfizer Officially Asks The FDA To Authorize Its COVID Vaccine For Kids Aged 5-11
By: Joe Hernandez | NPR
Posted on:
Updated October 7, 2021 at 9:27 AM ET WASHINGTON, D.C. (NPR) — Pfizer and BioNTech are officially asking the Biden administration to authorize the use of their COVID-19 vaccine for… Read More
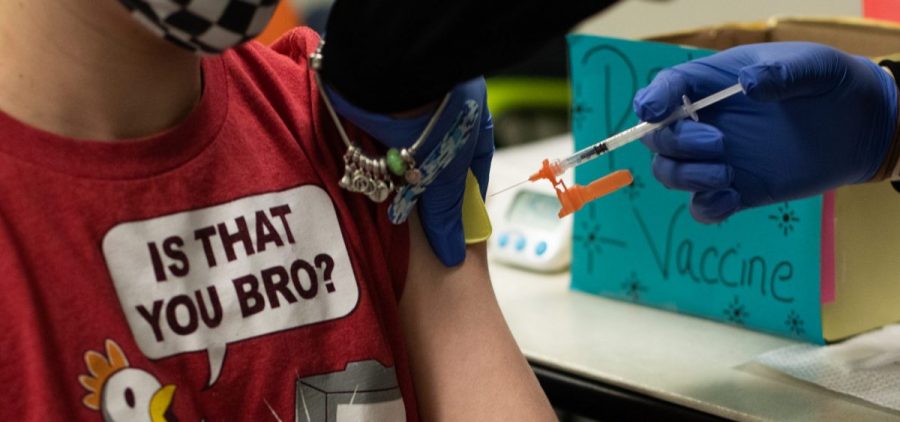
Pfizer Submits Favorable Initial Data To The FDA On Kids’ COVID-19 Vaccine Trial
By: Bill Chappell | NPR
Posted on:
WASHINGTON, D.C. (NPR) — Pfizer and BioNTech are another step closer to seeking authorization for young children to receive the COVID-19 coronavirus vaccine, submitting data to the Food and Drug… Read More
Ohio University Switching COVID-19 Vaccine In Response To Safety Concerns
By: WOUB News Team
Posted on:
ATHENS, Ohio (WOUB) — Ohio University is switching to the Pfizer COVID-19 vaccine for its student clinics after federal officials recommended a temporary halt in use of the Johnson &… Read More
Pfizer Says COVID-19 Vaccine Shows ‘100% Efficacy’ In Adolescents
By: Bill Chappell I NPR
Posted on:
Pfizer says it will submit the clinical trial results “as soon as possible” to the U.S. Food and Drug Administration, and is hoping to start vaccinating children before the next school year.
U.S. Reaches Deal With Pfizer For 100 Million More Vaccine Doses
By: Merrit Kennedy | NPR
Posted on:
The new deal effectively doubles the federal government’s order from the company. Pfizer said the U.S. government has agreed to pay $1.95 billion for the additional doses.

US Panel Endorses Widespread Use Of Pfizer COVID-19 Vaccine
By: Associated Press
Posted on:
WASHINGTON (AP) — A U.S. government advisory panel has endorsed Pfizer’s coronavirus vaccine, in a major step toward an epic vaccination campaign that could finally conquer the outbreak. The Food… Read More

Pfizer To Seek FDA OK For COVID-19 Vaccine ‘Within Days’
By: Joe Palca | NPR
Posted on:
The vaccine was found to be 95% effective in an updated study analysis. Safety data required by the Food and Drug Administration showed no serious concerns, the company said.

Pfizer Says Experimental COVID-19 Vaccine Is More Than 90% Effective
By: Joe Palca | NPR
Posted on:
Pfizer’s COVID-19 vaccine is the first to have data showing that it exceeded the minimum effectiveness threshold set by the Food and Drug Administration for emergency use.

U.S. To Get 100 Million Doses of Pfizer Coronavirus Vaccine In $1.95 Billion Deal
By: Sydney Lupkin | NPR
Posted on:
If the company’s vaccine candidate pans out, Americans can receive it for free, under the deal. The arrangement is part of the U.S. government’s push to have a vaccine widely available by January.

