You are viewing the "FDA" Archives

FDA faces pressure to act nationwide on red dye in food
By: Allison Aubrey | NPR
Posted on:
WASHINGTON (NPR) — There’s new pressure on the Food and Drug Administration to take action on the synthetic red dye after California passed a law to ban it last week…. Read More
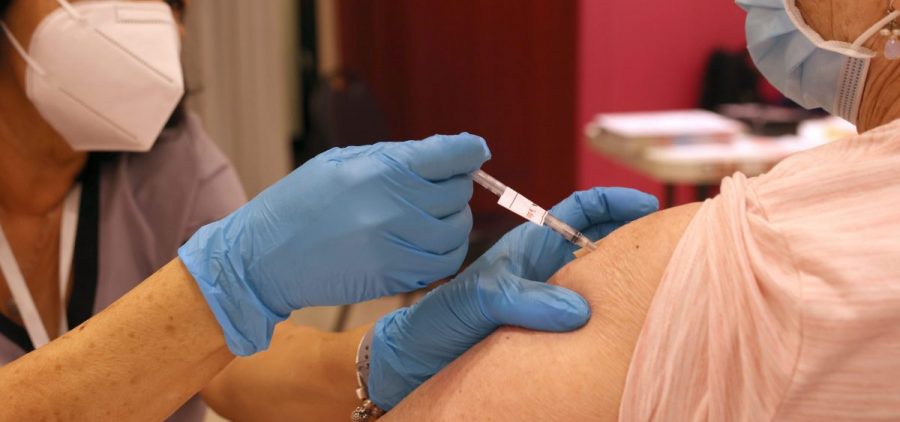
New COVID vaccines get FDA approval
By: Rob Stein | NPR
Posted on:
WASHINGTON (NPR) — The Food and Drug Administration approved a new round of vaccines against COVID-19. The vaccines from Moderna and Pfizer and its partner BioNTech were approved Monday for… Read More
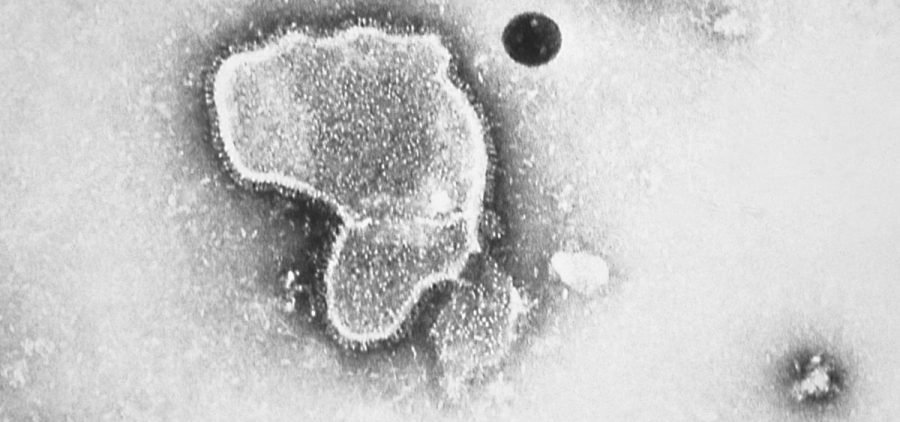
Pfizer’s RSV vaccine to protect babies gets greenlight from FDA
By: Sydney Lupkin | NPR
Posted on:
WASHINGTON (NPR) — The Food and Drug Administration has approved the first RSV vaccine for expectant mothers aimed at protecting their newborn babies. Given during the third trimester of pregnancy,… Read More
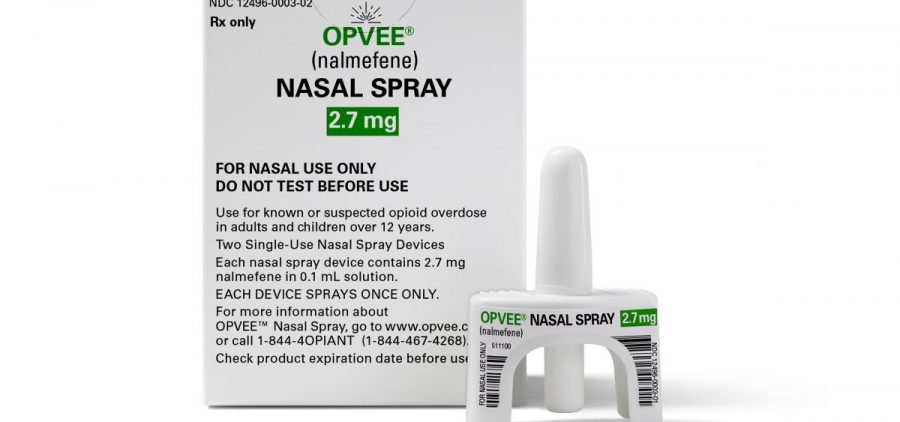
A new nasal spray to reverse fentanyl and other opioid overdoses gets FDA approval
By: Associated Press
Posted on:
WASHINGTON (AP) — U.S. health regulators on Monday approved a new easy-to-use version of a medication to reverse overdoses caused by fentanyl and other opioids driving the nation’s drug crisis…. Read More

What’s next for abortion pill legal battle as Supreme Court decision maintains access
By: PBS Newshour
Posted on:
WASHINGTON, D.C. (NewsHour) — The Supreme Court decided Friday to preserve access to the abortion drug mifepristone, for now. The pill will remain on the market while the Biden administration… Read More
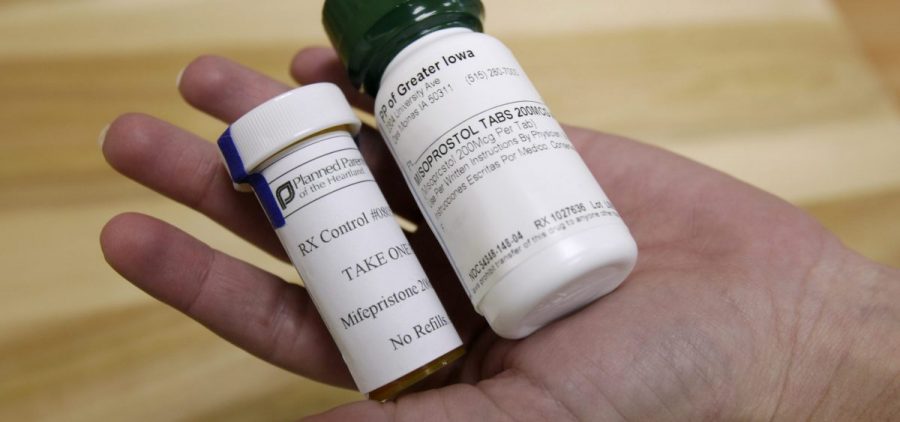
Conflicting rulings by federal judges leaves state of abortion pill in limbo
By: Geoff Bennett | Matt Loffman | PBS NewsHour
Posted on:
WASHINGTON (NewsHour) — Dueling decisions from federal judges over the FDA’s approval of mifepristone, a main abortion pill, mark the latest flash point in the fight over reproductive rights. The… Read More

Democratic state attorneys general sue the Biden administration over abortion pill rules
Updated February 24, 2023 at 4:02 PM ET WASHINGTON (NPR) — A coalition of state attorneys general is suing the Food and Drug Administration over abortion pill rules, accusing the… Read More

The FDA proposes new targets to limit lead in baby food
By: Allison Aubrey | Jane Greenhalgh | NPR
Posted on:
WASHINGTON, D.C. (NPR) — It’s not possible to remove all traces of lead from the food supply, because the heavy metal is found throughout the environment and can be absorbed… Read More
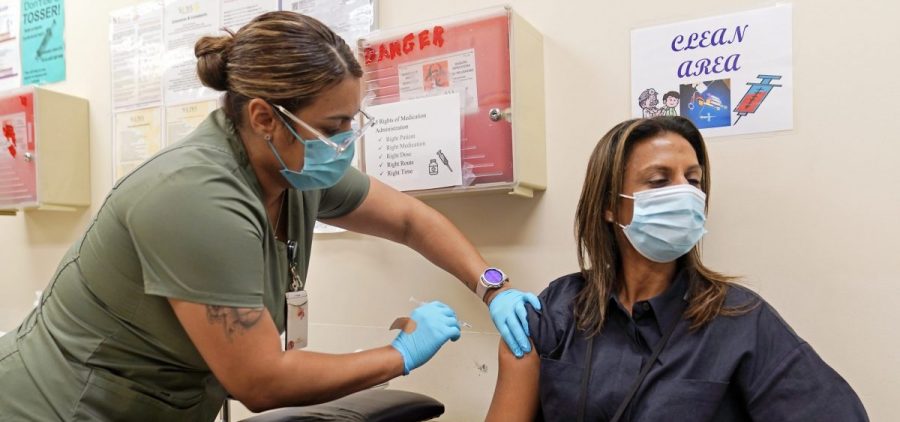
FDA considers major shift in COVID vaccine strategy
By: Rob Stein | NPR
Posted on:
Updated 10:30 a.m. ET WASHINGTON, D.C. (NPR) — The Food and Drug Administration is considering a major shift in the nation’s COVID-19 vaccine strategy. The goal is to simplify vaccination… Read More

Is lecanemab the Alzheimer’s drug that will finally make a difference?
By: Jon Hamilton | NPR
Posted on:
A drug that offers a small benefit to Alzheimer’s patients is making a big splash with doctors who treat the disease. The drug, a monoclonal antibody called lecanemab, dominated last… Read More

Thousands of toddler sippy cups and bottles are recalled over lead poisoning risk
By: Emma Bowman | NPR
Posted on:
WASHINGTON, D.C. (NPR) — Green Sprouts, a maker of reusable baby products sold at chain retailers including Whole Foods and Bed Bath & Beyond, is recalling its stainless-steel cups and… Read More

The FDA has officially declared a shortage of Adderall
By: Ayana Archie | NPR
Posted on:
WASHINGTON, D.C. (NPR) — The FDA has confirmed the nation is experiencing a shortage of Adderall after many pharmacies around the country have been unable to fill prescriptions and keep… Read More

FDA authorizes first revamp of COVID vaccines to target omicron
By: Scott Hensley | Rob Stein | NPR
Posted on:
WASHINGTON, D.C. (NPR) — The Food and Drug Administration authorized reformulated versions of the Moderna and Pfizer-BioNTech vaccines that aim to protect against the omicron variant. The new shots target… Read More
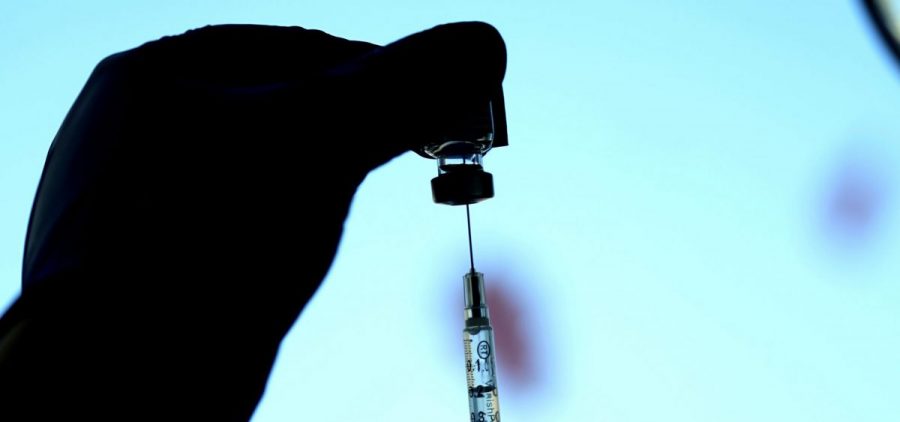
Pfizer asks FDA to greenlight new omicron booster shots, which could arrive this fall
By: Pien Huang | Will Stone | NPR
Posted on:
WASHINGTON, D.C. (NPR) — The U.S. is one step closer to having new COVID-19 booster shots available as soon as this fall. On Monday, the drugmakers Pfizer and BioNTech announced… Read More
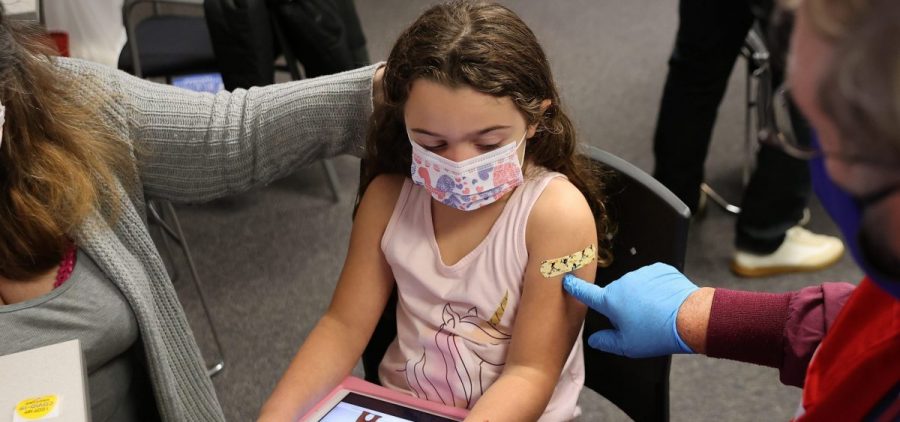
Advisers to the FDA back COVID vaccines for the youngest children
By: Scott Hensley | NPR
Posted on:
WASHINGTON, D.C. (NPR) — A committee of advisers to the Food and Drug Administration voted unanimously to recommend that the agency authorize COVID-19 vaccines from Moderna and Pfizer-BioNTech for children… Read More
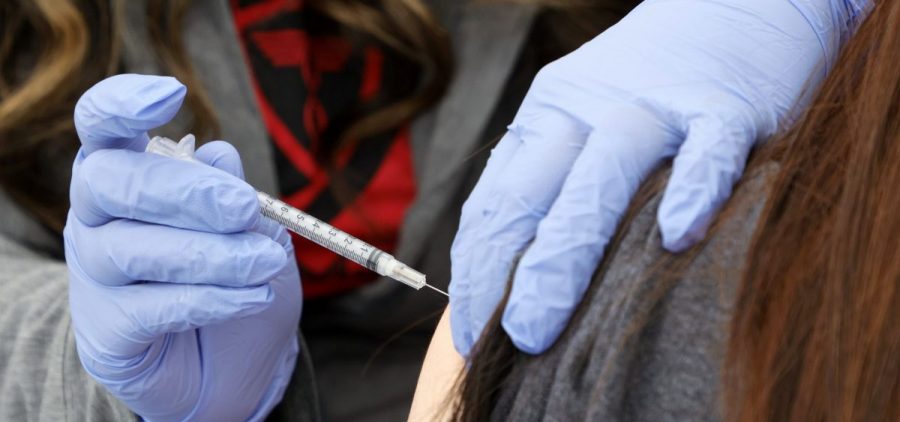
The FDA OKs another Pfizer or Moderna COVID booster for people 50 and up
By: Associated Press
Posted on:
WASHINGTON, D.C. (AP) — U.S. regulators are allowing people 50 and older to get another booster dose of the Pfizer or Moderna COVID-19 vaccine. The Food and Drug Administration’s decision aims… Read More
The FDA postpones a highly anticipated meeting on the Pfizer vaccine for young kids
By: Scott Hensley | NPR
Posted on:
WASHINGTON, D.C. (NPR) — A highly anticipated meeting of expert advisers to discuss whether to recommend the use of the Pfizer-BioNTech COVID-19 vaccine for young children has been postponed. The… Read More
COVID-19 vaccine for young kids could be ready this month
By: Peter Granitz | Rob Stein | NPR
Posted on:
Updated February 1, 2022 at 7:42 AM ET WASHINGTON, D.C. (NPR) — The last age group of the population unable to get a Covid-19 vaccine may soon be able to… Read More
FDA authorizes a Pfizer booster shot for children ages 12 to 15
By: Scott Hensley | Joe Hernandez | NPR
Posted on:
Updated January 3, 2022 at 10:41 AM ET WASHINGTON, D.C. (NPR) — The Food and Drug Administration has authorized the use of a Pfizer-BioNTech booster in adolescents 12 to 15… Read More

FDA authorizes 1st antiviral pill for COVID
By: Scott Hensley | NPR
Posted on:
WASHINGTON, D.C. (NPR) — In a highly anticipated decision, the Food and Drug Administration authorized the first antiviral pill to treat COVID-19 at home. The pill, called Paxlovid, is made… Read More
The FDA authorizes COVID booster shots for all U.S. adults
By: Scott Neuman I NPR
Posted on:
WASHINGTON, D.C. (NPR) — The Food and Drug Administration has given its OK for fully vaccinated Americans who are age 18 and older to receive a COVID-19 booster shot. The… Read More
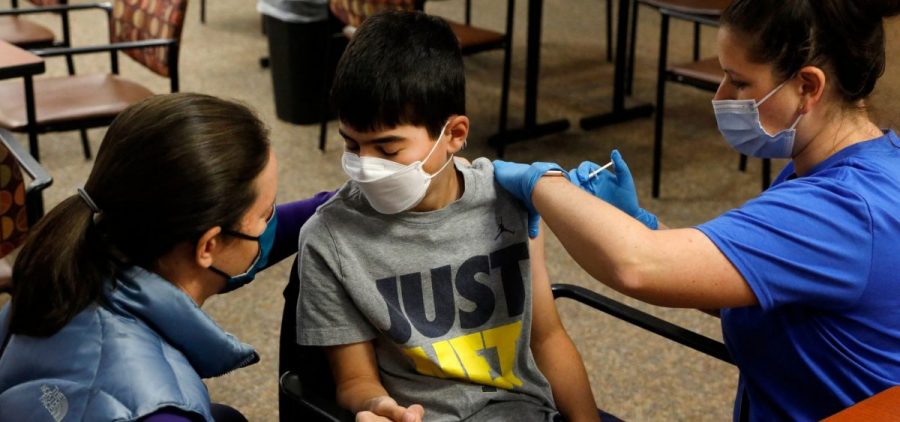
FDA authorizes use of Pfizer’s COVID vaccine for 5- to 11-year-olds
By: Joe Neel | NPR
Posted on:
WASHINGTON, D.C. (NPR) — The Food and Drug Administration has authorized a Pfizer-BioNTech COVID-19 vaccine for children ages 5 to 11. This lower-dose formulation of the companies’ adult vaccine was… Read More
Pfizer Officially Asks The FDA To Authorize Its COVID Vaccine For Kids Aged 5-11
By: Joe Hernandez | NPR
Posted on:
Updated October 7, 2021 at 9:27 AM ET WASHINGTON, D.C. (NPR) — Pfizer and BioNTech are officially asking the Biden administration to authorize the use of their COVID-19 vaccine for… Read More
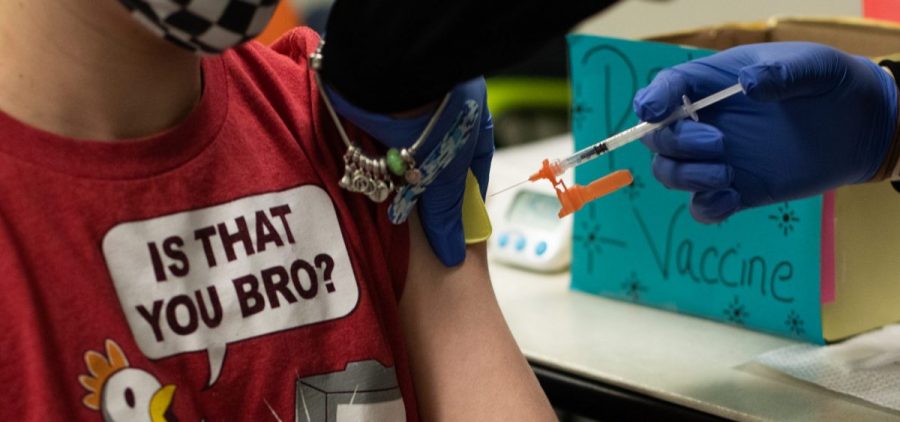
Pfizer Submits Favorable Initial Data To The FDA On Kids’ COVID-19 Vaccine Trial
By: Bill Chappell | NPR
Posted on:
WASHINGTON, D.C. (NPR) — Pfizer and BioNTech are another step closer to seeking authorization for young children to receive the COVID-19 coronavirus vaccine, submitting data to the Food and Drug… Read More
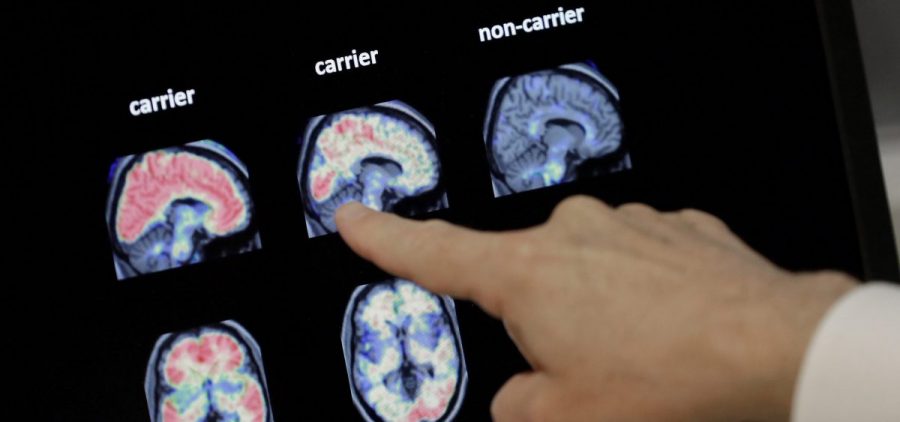
The FDA Has Approved A New Alzheimer’s Drug — Here’s Why That’s Controversial
By: Scott Hensley | Jon Hamilton | Laurel Wamsley | NPR
Posted on:
This is the first new drug approved for Alzheimer’s disease since 2003. It’s the first to show significant progress against the sticky brain plaques that are the hallmark of Alzheimer’s disease.

